H2O + Electrolytes
So last weekend, I noticed that there weren’t any Gatorade stands in my 5-mile race. I used to think that it had something to do with the race organizers having to arrange the right kind sponsorship. But, I’m pretty sure they didn’t have it either at the last 4-mile race I attended and even my most recent 10K. Although… there were Gatorade stands along the entire course of the Brooklyn Half Marathon. Why is that?
I’m speculating, but I’m guessing it has something to do with the distance of the course. I think anything shorter than a half marathon will only have water stations, otherwise, they’ll either distribute energy gels or gatorade. And, there’s probably a good reason why!
For one, you spend less time sweating overall when you run a shorter race than a long one so, technically, your chances of becoming dehydrated should be less (assuming that the weather is cooperating and you take advantage of the water stations). Whereas, if you run at least the distance of a half marathon, the rate at which you’re losing water through sweat is more likely to be greater than your water intake; it’s also easy to lose track of how much you’ve had to drink when you’re tired and just want to finish as quickly as possible. Therefore, you need a quicker way to rehydrate yourself when you’re running longer distances (especially when you’re training in the heat). This is where electrolytes come in.
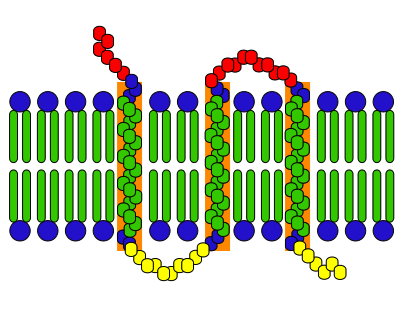
Electrolytes are substances that dissolve in a solution into electrically charged ions. Their main function is to maintain fluid balance and help transmit nerve impulses and muscular activity (also known as ‘cotransporters’ – they assist in moving things in and out of a cell membrane). Because electrolytes are mostly composed of Sodium (Na), Potassium (K), and Chloride (Cl), they also moderate the water distribution between cellular compartments.
To put it simply, sweat is hypertonic (concentrated solute). When we sweat, we lose about equal amounts of Sodium and Chloride and a little less of Potassium. If we lose too much of these substances in our system, it throws off our fluid balance and the trasmission of nerve impulses and muscle activity. Not to mention, you’ll feel really dehydrated!
The problem, in this case, with just drinking water is that you’re not replacing the electrolytes that you’re losing rapidly. Drinking water will get reabsorbed into your system and that’s good, but you won’t be performing as well as you would unless you were putting the electrolytes back in. I’m thinking I’ll bring my own Gatorade for this upcoming weekend’s 5-mile run. I’m curious to see if it makes much of a difference.
If your curious to read more, here’s a pretty good brief article on it from Discovery Health – “What are electrolytes?”
Correction: courtesy of my friend Johan at Stanford Med School – aka genius scientist 🙂
There is something that a lot of sources get wrong about sweat though: it is HYPOtonic as opposed to an HYPERtonic salt solution, when produced. When you drink Gatorade, you are replacing both the fluid and the salt that was lost via sweating. Gatorade does not taste salty partly because the concentration of electrolyte in it is less than that in the blood. Oh btw, they also add glucose in it to help the electrolytes get in the the cells easier. Anyway, that is all to say that sweat, when produced, is hypotonic (proportionally more water was lost from the body as compared to salt) and only when the water evaporates can the excess salt be considered HYPERtonic. If produced sweat was hypertonic, your Gatorade would probably taste like salt-water and be a lot sweeter at the same time too! It would taste really bad.